Author: Omer Hassan Jamy, MD1, Nicholas Coombs, PhD, MSTAT2, Debra Wujcik, PhD3, Aaron Galaznik, MD3
1University of Alabama at Birmingham, ,2Piedmont Research Strategies, Inc., 3Carevive Systems, Inc.
Introduction
Prior research has demonstrated the benefit of gathering patient-reported outcomes (PROs) in oncology practice for improved clinical outcomes, health resource utilization and quality of life. The FDA guidance on Core Patient Reported Outcomes in Cancer Clinical Trials further highlights the importance of PROs for capturing disease, treatment, and functional impacts of oncology therapy. For patients with Acute Myeloid Leukemia (AML), disease mortality and survey compliance pose structural challenges to gathering PRO data. This study was conducted to address this need.
Aim
- To demonstrate the feasibility of gathering PROs with real world data among a cohort of patients with AML using a remote symptom monitoring platform across baseline prognostic and therapeutic factors
- To describe the impact of cytogenetic risk (Poor vs. Not-Poor) and treatment type (Curative vs. Non-Curative) on functional status.
- Prognostic risk was determined using cytogenetics, and molecular abnormalities. Poor risk was classified using patients with the following: Poor-risk by FISH analysis, mutations in TP53, RUNX1 and ASXL1 and secondary AML
- Curative Therapy included intensive induction, consolidation and allogeneic stem cell transplantation
Methods
Study Design
Feasibility study with a prospective cohort and real-world data
Participants
- Patients aged 18 or older currently receiving treatment for AML
- Access to Smart phone, tablet, or computer
Instrument
Patient Reported Outcomes Mobile Platform (PROmpt®), an application for remote patient reporting and provider symptom management
Data Collection
- After consent, patients were enrolled in PROmpt®| and completed baseline surveys
- Weekly assessments included symptom burden (PRO-CTCAE) and function impact (PROMIS 4A)
- Clinical, biomarker, and treatment data were obtained from electronic medical record structured data
Analysis
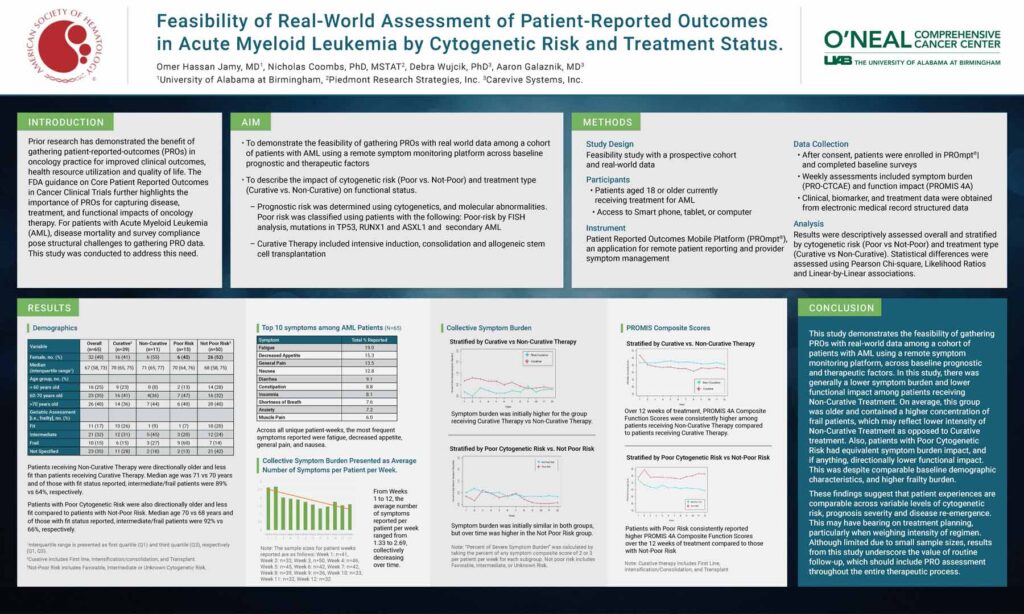
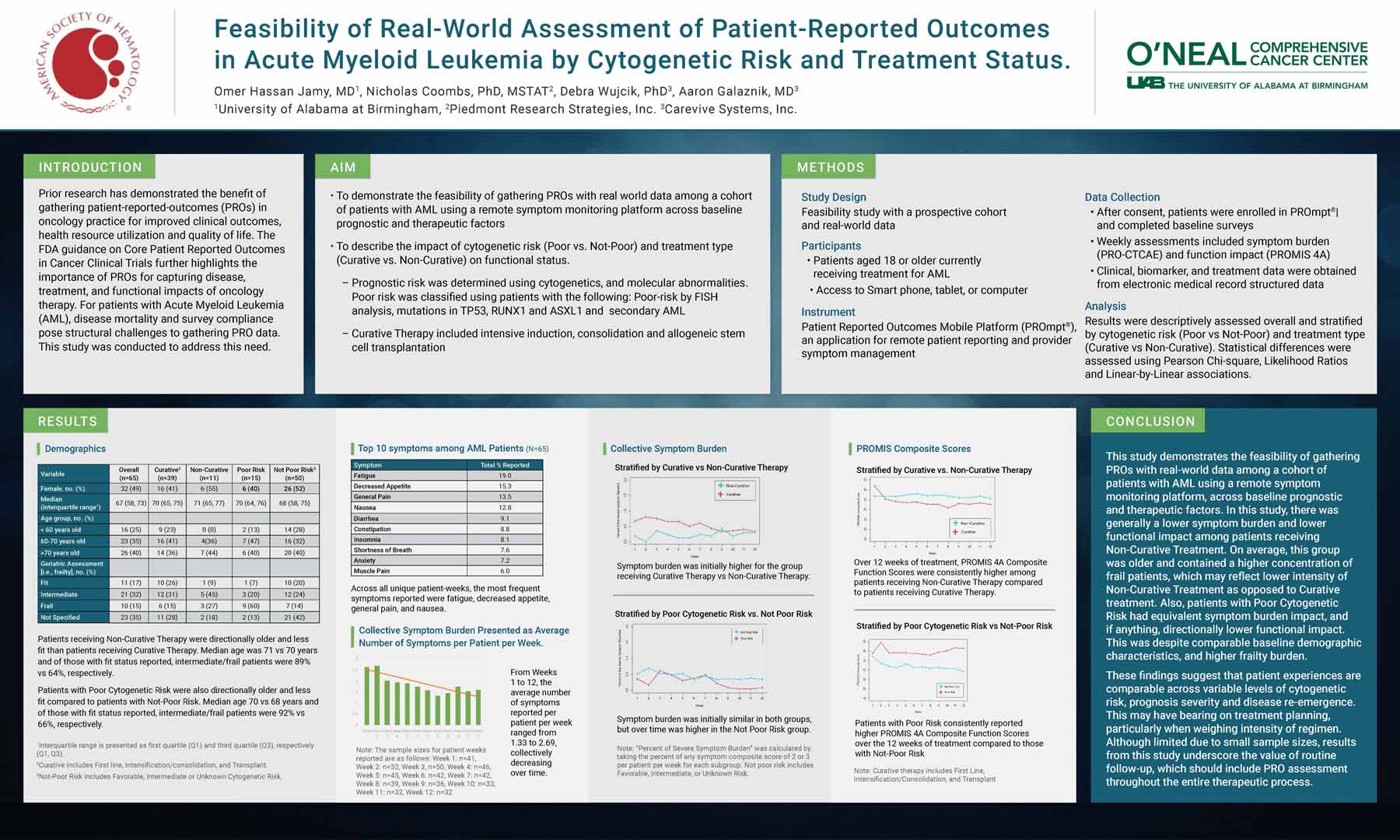
Results were descriptively assessed overall and stratified by cytogenetic risk (Poor vs Not-Poor) and treatment type (Curative vs Non-Curative). Statistical differences were assessed using Pearson Chi-square, Likelihood Ratios and Linear-by-Linear associations.
Conclusion
This study demonstrates the feasibility of gathering PROs with real-world data among a cohort of patients with AML using a remote symptom monitoring platform, across baseline prognostic and therapeutic factors. In this study, there was generally a lower symptom burden and lower functional impact among patients receiving Non-Curative Treatment. On average, this group was older and contained a higher concentration of frail patients, which may reflect lower intensity of Non-Curative Treatment as opposed to Curative treatment. Also, patients with Poor Cytogenetic Risk had equivalent symptom burden impact, and if anything, directionally lower functional impact. This was despite comparable baseline demographic characteristics, and higher frailty burden.
These findings suggest that patient experiences are comparable across variable levels of cytogenetic risk, prognosis severity and disease re-emergence. This may have bearing on treatment planning, particularly when weighing intensity of regimen. Although limited due to small sample sizes, results from this study underscore the value of routine follow-up, which should include PRO assessment throughout the entire therapeutic process.