Authors: Ethan Basch, MD1, Huamao M. Lin, PhD2, Mary Lynn Cala, MS2, Meni Styliadou, LLM2, Aaron Galaznik, MD3, Debra Wujcik, PhD, RN3, Emelly Rusli, MPH3, Nicholas Coombs, PhD, MSTAT4, Emily Beamon, PhD, MA, MPH4
1University of North Carolina, 2Takeda Pharmaceuticals, 3Carevive Systems, Inc., 4Piedmont Research Strategies
Background
- Prior studies have shown the value of Patient-Reported Outcomes (PRO) in cost burden, quality of life (QoL), and survival in cancer care,1-4.
- Technology advances allow capture of PRO data from home using devices such as smart phones, sending PRO results directly to the electronic medical record (EMR), allowing clinical professionals to monitor patient-reported status in real time and longitudinally.
- To facilitate focus on patient-centricity and value-based care, the European Health Outcomes Observatory (H2O) developed a Core Outcomes Set (COS) in lung cancer5.
- We explored the feasibility of deploying standardized PRO measures among patients with lung cancer in US clinical practice.
Methods
- A retrospective database study, with IRB waiver, was conducted in 207 adult lung cancer patients on active treatment across 9 US cancer centers from 9/2020 to 5/2023 using Carevive PROmpt®, an electronic PRO (ePRO) remote symptom monitoring platform with weekly surveys comparable in scope to H2O COS using:
- The PRO-CTCAE to assess symptomatic adverse events
- The PROMIS 4A Physical Function to assess physical function – Items 29 and 30 from the EORTC QLQ-C30 to assess global health status/quality of life
- Moderate and severe PRO-CTCAE symptoms scores triggered alerts to the clinical team (e.g., nurses, Advanced Practitioner nurses (AP), and physicians).
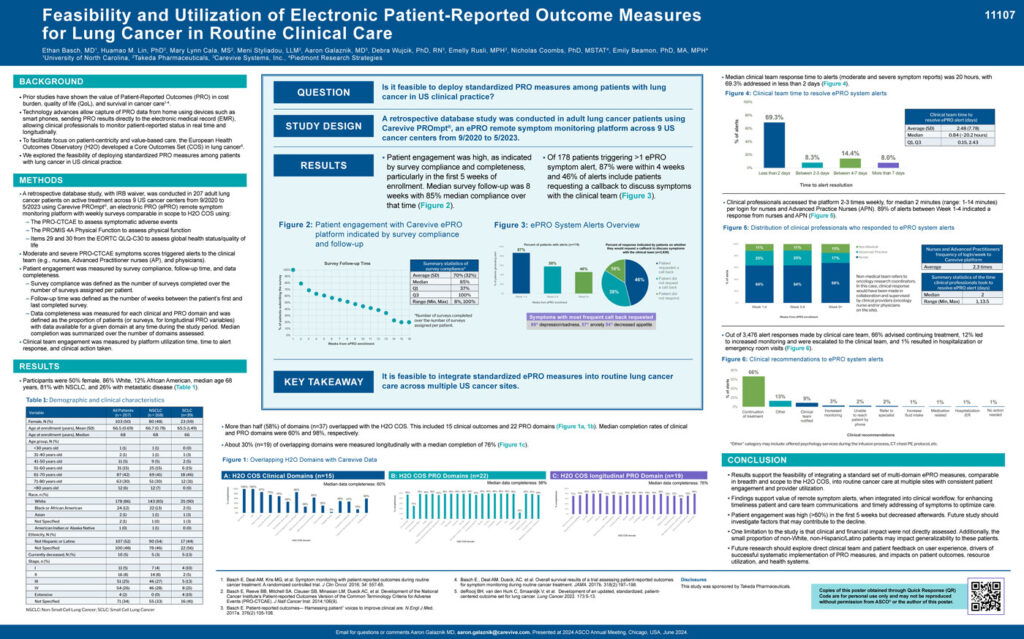
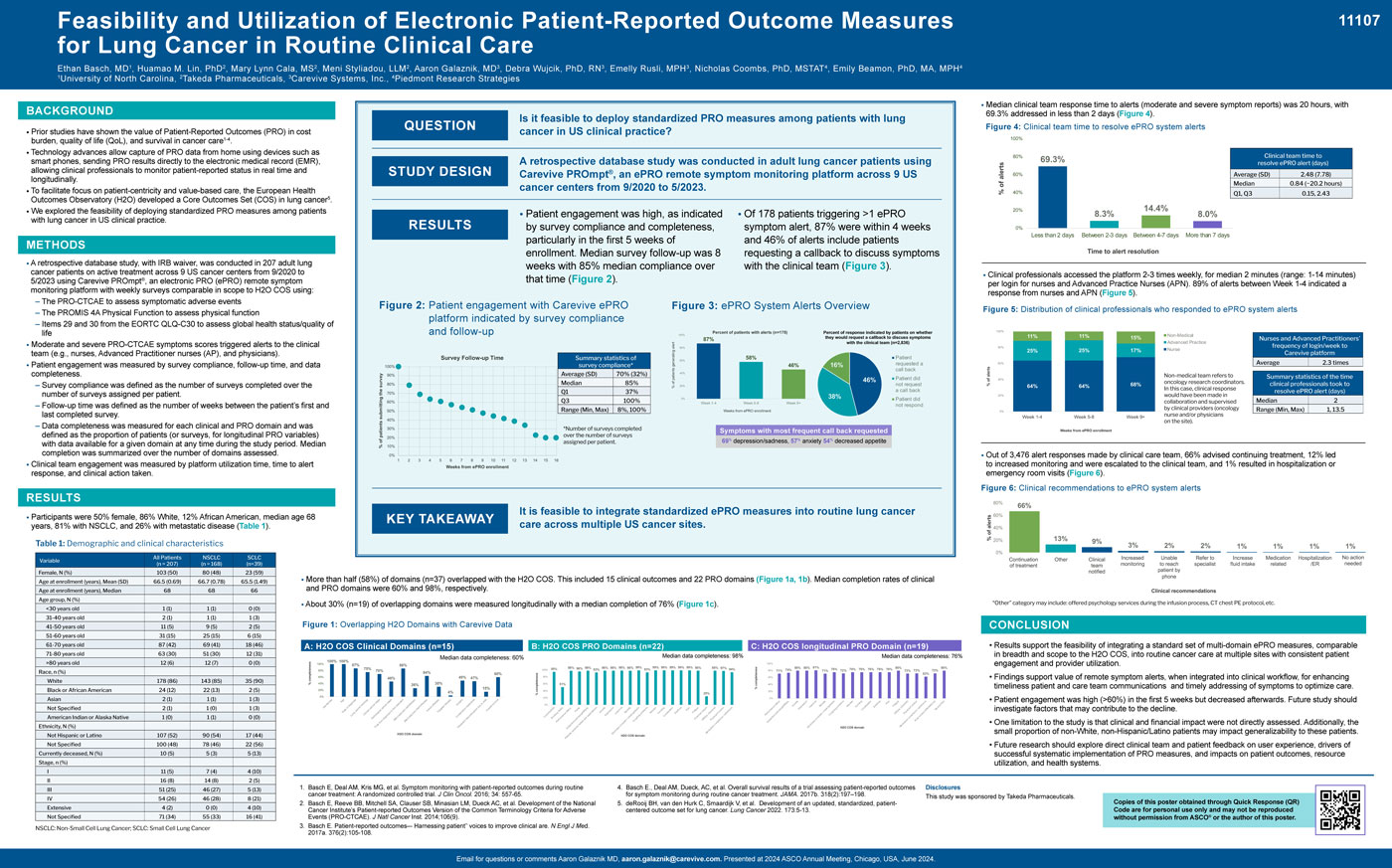
- Patient engagement was measured by survey compliance, follow-up time, and data completeness.
- Survey compliance was defined as the number of surveys completed over the number of surveys assigned per patient.
- Follow-up time was defined as the number of weeks between the patient’s first and last completed survey. – Data completeness was measured for each clinical and PRO domain and was defined as the proportion of patients (or surveys, for longitudinal PRO variables) with data available for a given domain at any time during the study period. Median completion was summarized over the number of domains assessed.
- Clinical team engagement was measured by platform utilization time, time to alert response, and clinical action taken.
Conclusion
- Results support the feasibility of integrating a standard set of multi-domain ePRO measures, comparable in breadth and scope to the H2O COS, into routine cancer care at multiple sites with consistent patient engagement and provider utilization.
- Findings support value of remote symptom alerts, when integrated into clinical workflow, for enhancing timeliness patient and care team communications and timely addressing of symptoms to optimize care.
- Patient engagement was high (>60%) in the first 5 weeks but decreased afterwards. Future study should investigate factors that may contribute to the decline.
- One limitation to the study is that clinical and financial impact were not directly assessed. Additionally, the small proportion of non-White, non-Hispanic/Latino patients may impact generalizability to these patients.
- Future research should explore direct clinical team and patient feedback on user experience, drivers of successful systematic implementation of PRO measures, and impacts on patient outcomes, resource utilization, and health systems.
1. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016; 34: 557-65.
2. Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s Patient-reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9).
3. Basch E. Patient-reported outcomes–- Harnessing patient’’ voices to improve clinical are. N Engl J Med. 2017a. 376(2):105-108.
4. Basch E., Deal AM, Dueck, AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017b. 318(2):197–198.
5. deRooij BH, van den Hurk C, Smaardijk V, et al. Development of an updated, standardized, patientcentered outcome set for lung cancer. Lung Cancer 2022. 173:5-13.