Authors: Aaron Galaznik, MD1, Emelly Rusli, MPH1, Tracy Holt, MS, MPH2, Mordecai Kramer, MBA1, Peter Trask, PhD MPH3
1Carevive Systems Inc, 2Parexel, 3Genentech
Background
- In breast cancer treatment, research has identified racial differences in the ability to receive the full dose of prescribed chemotherapy which may be due in part to cancer-treatment symptom burden.1
- In clinical trials, evaluation by race is challenging due to enrollment disparities, with African Americans (AA) accounting for 7% of trial participants, but comprising 12% of the US total population.
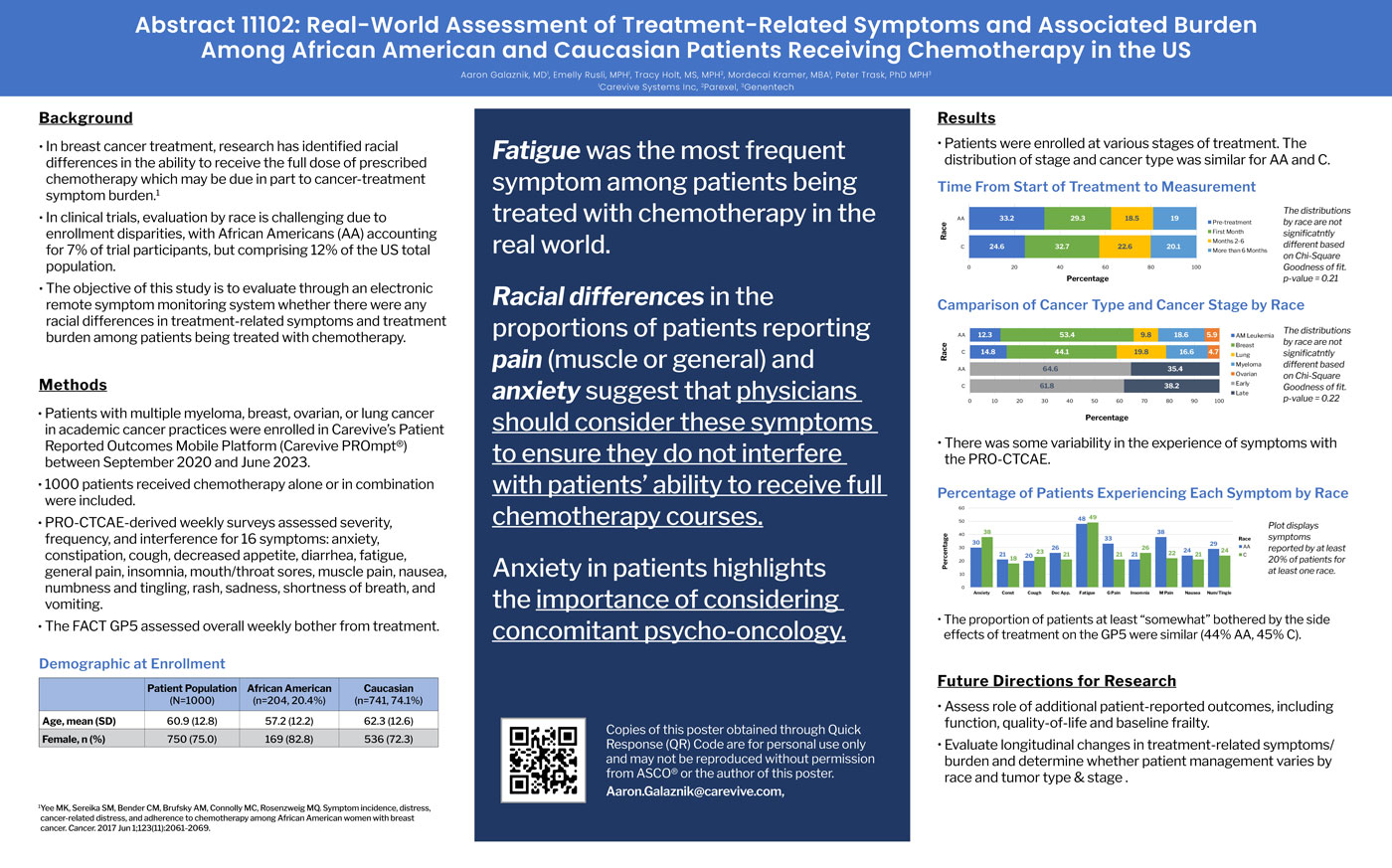
- The objective of this study is to evaluate through an electronic remote symptom monitoring system whether there were any racial differences in treatment-related symptoms and treatment burden among patients being treated with chemotherapy.
Methods
- Patients with multiple myeloma, breast, ovarian, or lung cancer in academic cancer practices were enrolled in Carevive’s Patient Reported Outcomes Mobile Platform (Carevive PROmpt®) between September 2020 and June 2023.
- 1000 patients received chemotherapy alone or in combination were included.
- PRO-CTCAE-derived weekly surveys assessed severity, frequency, and interference for 16 symptoms: anxiety, constipation, cough, decreased appetite, diarrhea, fatigue, general pain, insomnia, mouth/throat sores, muscle pain, nausea, numbness and tingling, rash, sadness, shortness of breath, and vomiting.
- The FACT GP5 assessed overall weekly bother from treatment.
Future Directions for Research
- Assess role of additional patient-reported outcomes, including function, quality-of-life and baseline frailty.
- Evaluate longitudinal changes in treatment-related symptoms/ burden and determine whether patient management varies by race and tumor type & stage .
- Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017 Jun 1;123(11):2061-2069.