Authors: Jasmeen Kaur1, Emelly Rusli, MPH1, Debra Wujcik, PhD1, Aaron Galaznik, MD1
1 Carevive Systems, Inc.
Objectives
Cyclin-Dependent Kinase Inhibitors (CDKI) are an important advancement in the treatment of Metastatic Breast Cancer. Barriers to clinician adoption, such as tolerability concerns, may limit front line use. The objective of this study is to use electronic patient-reported outcome (ePRO) and a remote symptom monitoring platform to evaluate symptom differences among late-stage breast cancer patients with and without CDKI treatment.
Methods
- Carevive’s Patient Reported Outcomes Mobile Platform (Carevive PROmpt®) was used by late-stage breast cancer patients for reporting symptoms between September 2020 and June 2022.
- Human epidermal growth factor receptor (HER2) positive patients were excluded, leaving a total of 84 patients.
- Weekly survey results were used to assess symptom incidence at baseline, then weeks 4, 8,
12 and 16. Questions derived from the PRO-CTCAE assessed severity, frequency, and interference for 15 symptoms: Anxiety, Constipation, Decreased Appetite, Diarrhea, General Pain, Insomnia, Nausea, Numbness and Tingling, Shortness of Breath, Vomiting, Fatigue, Mouth/Throat Sores, Muscle Pain, Rash, and Sadness. - Baseline demographics and characteristics were assessed at baseline and stratified by CDKI use.
- The association of CDKI’s with symptoms in late- stage breast cancer patients was examined using GEE (Generalized Estimating Equation) logistic regression model after adjusting for age.
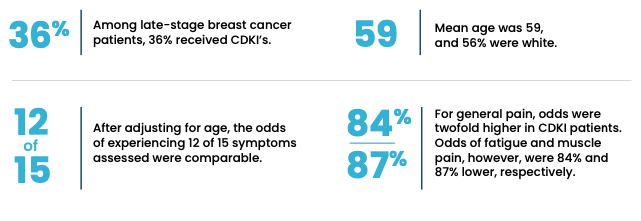
Results
 Conclusions
Conclusions
This study found that only one-third of late-stage breast cancer patients were prescribed CDKI’s. Symptom experience was overall comparable between groups. Overall study results support tolerability of CDK inhibitors in late-stage patients, and furthermore highlights how ePRO and remote symptom monitoring solutions can support management of patient symptoms while on treatment

 Conclusions
Conclusions