Carevive is recruiting institutions to participate in the following research studies.
Benefits of participation include:
- Authorship on study-related abstracts/manuscripts
- Access to study data as preliminary data for future grant applications
- Discounted use of our commercial licenses
If you have an idea or suggestion to implement Carevive in a solution, get in touch with our team today!
Evidence-Based Clinical Practices and Shared Decision Making for HER2+ Metastatic Breast Cancer
Purpose: Four cancer networks across the United States who are participating in the CMS’s Oncology Care Model (OCM) payment program will be selected to participate in this educational/research initiative. All organizations participating in the OCM program will be invited. At initiation, Carevive will work with these institutions and their population health vendor/data warehouses to better understand what are their providers’ metastatic breast cancer (MBC) practice patterns and adherence to evidence-based guidelines. Following the CME, the medical oncologists treating HER2+ MBC patients will use the Carevive software, which includes an electronic Patient-Reported Outcomes (e-PRO) platform for patients and treatment planning tools for clinicians. The Carevive software interfaces with most enterprise EHRs. Through the e-PRO platform, treating clinicians can better understand patients’ personalized needs that impact treatment planning.
Objectives:
- Aim 1: To explore the impact of educational/clinical technology interventions on oncology providers’ adherence to evidence-based treatment recommendations for patients with HER2+ MBC.
- Aim 2: To describe real-world treatment decisions for patients with HER2+ MBC. We will aggregate and analyze patient-reported and clinical factors associated with treatment decisions for HER2-positive MBC.
- Aim 3: To describe real-world clinician practice patterns of medical oncologists managing older patients with HER2+ MBC, including sub-analyses of practice patterns for those who are considered “fit”, “intermediate fit”, and “frail”.
Emerging Therapies and Clinical Trial Opportunities for ER-Positive, HER2-Negative Metastatic Breast Cancer and HER2-Positive Breast Cancer
Purpose: Clinical trial participation is needed to further the development of effective therapeutic agents for breast cancer, yet low participation rates persist. Providers and patients currently lack effective tools to identify appropriate clinical trials, promote communication about clinical trial participation, overcome attitudinal barriers and eliminate knowledge gaps. Using a pre-test post-test design, this study will evaluate the impact of a novel care planning system, the Carevive Care Planning System (CPS) on patient and provider knowledge, beliefs/attitudes, and behaviors around breast cancer clinical trial participation.
Objectives: The primary objectives of this study are: 1) To evaluate the impact of the intervention (Carevive CPS + clinical training) on patient and oncology provider knowledge, attitudes and beliefs about breast cancer clinical trials, and 2) To evaluate the impact of the intervention on patient referral to and enrollment in breast cancer clinical trials.
Request ParticipationUnderstanding Evidence-Based Practice Patterns in Advanced Non-Small Cell Lung Cancer (NSCLC)
Purpose: In this project, three cancer networks will be selected to participate in this educational/research initiative. At initiation, Carevive will work with these institutions and their population health vendor/data warehouses to better understand what are their providers’ NSCLC practice patterns and adherence to evidence-based guidelines. Providers from these centers will participate in a half-day, accredited educational conference with NSCLC expert faculty to discuss the NSCLC treatment landscape. Following the conference, the medical oncologists treating advanced NSCLC patients will use the Carevive software, which includes an electronic Patient-Reported Outcomes (e-PRO) platform for patients and treatment planning tools for clinicians.
Request ParticipationObjectives: The goals of this initiative are to close clinical knowledge and performance gaps by providing oncology clinicians with the latest advances and emerging research in the evidence-based and personalized treatment of advanced NSCLC patients. Also, the goal is to offer expert insights, IT infrastructure and clinical workflow processes to support cancer center’s need to meet quality measures relevant to value-based care delivery to patients with advanced NSCLC. We also hope gain insights on clinician practice patterns related to advanced NSCLC, and the correlation between advanced NSCLC patients’ reported goals of care and advanced NSCLC patients’ fit/frailty status and treatment decisions.
Purpose: This study will explore the feasibility, use, and utility of incorporating an electronically completed geriatric assessment (GA) that evaluates a patient’s fit/frailty status, and exploring its influence on treatment decision-making.
Using the Carevive electronic patient reported outcomes platform (Carevive e-PRO), 120 patients (30 at each of four sites) with multiple myeloma who at a treatment decision-making point will complete the validated GA (Palumbo et al.), and the results of this assessment will be presented back to the clinician at the point of care for consideration when making treatment decisions.
Objectives: The primary objective of this project is to evaluate the feasibility and usability of incorporating a brief electronic geriatric assessment into routine clinical care for older adults with multiple myeloma, and to evaluate physician use of and perceived utility of the geriatric assessment, including use and utility of the GA to facilitate treatment decision-making. A secondary objective is to describe real-world treatment decision-making patterns of hematologists managing elderly patients with Multiple Myeloma, including sub-analyses of these decisions for those who are considered “fit”, “intermediate fit”, and “frail”.
Request ParticipationUnderstanding Evidence-Based Practice Patterns in Advanced NSCLC: An Educational/Research Initiative in Mid-Atlantic States
The goals of this initiative are to close clinical knowledge and performance gaps by providing oncology clinicians with the latest advances and emerging research in the evidence-based and personalized treatment of advanced NSCLC patients. Also, the goal is to offer expert insights, IT infrastructure and clinical workflow processes to support cancer center’s need to meet quality measures relevant to value-based care delivery to patients with advanced NSCLC. We also hope to gain insights on clinician practice patterns related to advanced NSCLC, and the correlation between advanced NSCLC patients’ reported goals of care and advanced NSCLC patients’ fit/frailty status and treatment decisions.
Achievement of National Quality Standards in Breast Cancer: A Pilot Study on the Impact of Certified Education Plus a Novel Care Planning Tool
The overall goal of this study is to evaluate the impact of the Carevive Care Planning System (CPS) technology and accredited continuing medical education on cancer center adherence to quality metrics relevant to the management of breast cancer patients (developed by ASCO and ONS). Five cancer centers will participate in the study with each site recruiting breast cancer patients. Clinicians will participate in the accredited continuing medical education and then, along with their respective patient participants, interact with the Carevive CPS™ during two visits, 6-weeks apart, with patients receiving a care plan at each visit. Provider’s patient-level adherence to 31 specific quality metrics will be evaluated by chart review at pre-test (baseline) and post-test (12 weeks), complemented by specific data extracted from the Carevive CPS platform.
Improving Clinical Trial Awareness in Non-Small Cell Lung Cancer: Pilot Testing a Novel Health Care Information Technology Platform Plus Certified Training at the Point of Care
The overall goal of the lung cancer trials study is to evaluate the impact of a novel individualized care planning/educational technology, the Carevive Care Planning System (CPS), on referral to clinical trials for recurrent or metastatic lung cancer, and patient and provider knowledge, attitudes, and beliefs about clinical trials. The study will enroll 30 patients per site over approximately six months, each of whom will participate in the CPS two-part intervention to consist of (1) clinician participation in certified training (CME) about the role of biomarkers in NSCLC treatment and new targeted and immunotherapy agents for NSCLC, and (2) use of the CPS at the point of care to identify clinical trial opportunities for patients with NSCLC interested in learning about trial participation as a treatment option, and providing personalized education to patients about trials for which they are eligible. Study outcomes will be evaluated at baseline and 6 weeks using questionnaires adapted from the literature.
Improving the Quality of Multiple Myeloma Treatment with Patient Care Plans: Pilot Testing the Impact of Certified Training and Care Planning on Symptom Management
The overall goal of the myeloma study is to evaluate the use of the Carevive Care Planning System (CPS) technology and certified training (CME) on supportive care and symptom management practices for individuals with myeloma receiving active treatment. Five cancer centers will participate in the study and will recruit 15-30 patients per site over a 6-month period. Each patient participant and his/her provider(s) will interact with the Carevive CPS during two visits, with patients receiving a personalized care plan at each visit.
Improving Surgical/Medical Oncology Collaboration for Breast Cancer Treatment Planning: Pilot Testing the Impact of Certified Training and Patient Care Planning
Neoadjuvant systemic therapy is vastly underutilized in eligible patients desiring breast conservation. Provider and institutional factors, such as provider knowledge and institutionally based care coordination networks, likely play large roles in influencing treatment patterns. In addition, patient factors such as distress and lack of information may cloud patient decision-making capabilities at this time. In this study, six cancer programs will accrue 35 newly diagnosed breast cancer patients to participate in a study over a 3-month period (n=105 breast cancer patients total). Patients will be recruited from surgical practices and will be referred to medical oncology, where they will receive a care plan that outlines initial treatment, as well as distress screening and management.
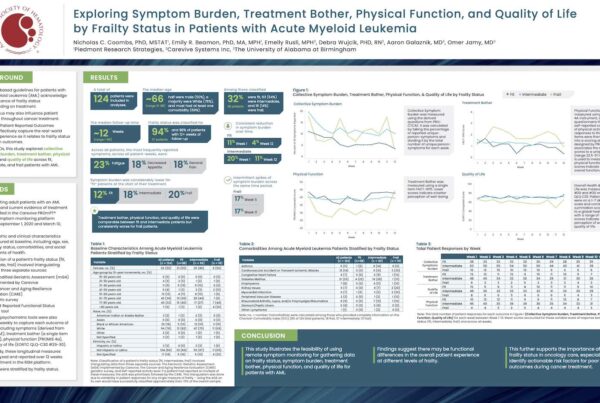
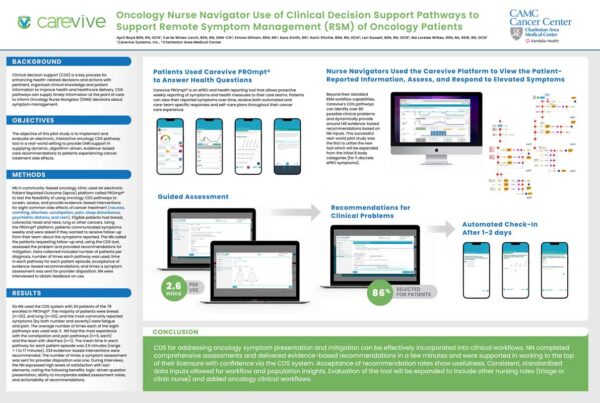
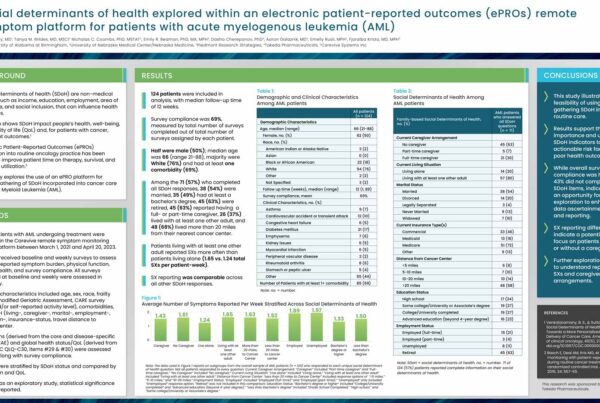
Relevant poster presentations from past research studies: